202510 Bennett Malbon Resume.pdf

Role : Co-Inventor, Chief Product & Technology Officer
When : Full Time 2014-2018, Part Time 2019-2023
Summary
Novaseek’s SaaS acted as virtual biobank using hospital EMR data to match remnant biospecimens to research projects. Researchers were able to create cohorts describing the clinical characteristics of patients (ie demographics, diagnosis, medications, lab results, pathology) and the software would algorithmically match remnant blood or other specimen types in the clinical lab, triggering a logistics process to de-identify and ship matching specimens to researchers. As co-inventor and founding product & technology officer, Bennett led the ground-up development of this product and oversaw deployment to 16 acute care hospitals, serving orders to most of the top 10 pharma and many diagnostic developers.


Value Proposition
Researchers
Researchers got access to fresh specimens with rich longitudinal Real World Data about the donor. Compared to traditionally banked specimens, these specimens were never frozen, serial collections from the same donor over time were available, and the longitudinal data set supported rich clinical insights into the donor and cohort criteria not frequently available from traditional biobanks.
Hospitals
Hospitals got a novel new revenue stream from selling specimens to researchers, and were able to meaningfully contribute to biomedical research even if they were a small to mid-sized hospital that did not traditionally support research programs.
Monetization
Novaseek charged a per-specimen fee to the researcher which covered Novaseek’s costs (shipping kits, sales, operations), payment to the hospital, and profit for Novaseek. This model had a principal challenge where every project was a one-off and most revenue had an associated Cost of Sales and project start-up that significantly cut into margins.
Challenges
Bridging hospitals and life sciences presented some unique challenges!
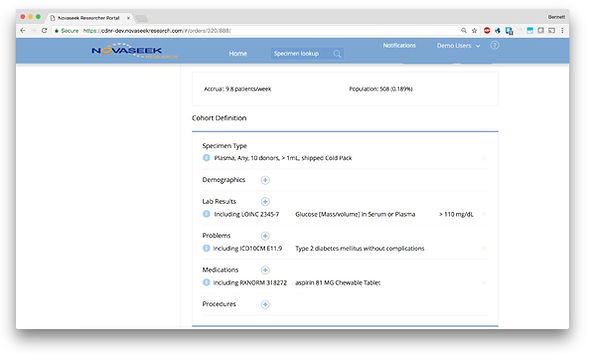
Sales and Onboarding
One of the first lessons learned was that researchers are naive of medical coding standards used by EMRs. For instance, they ask for “Lyme Disease” which could be an ICD10 diagnosis code or a positive lab result with multiple test options. Since we frequently found ourselves translating as part of the sales process, we built a cohort search tool to make it easier to see the different coding variants associated with lay nomenclature, and see prevalence in the Real-World-Data so that users were guided to pick the most relevant EMR codes to find their patients. The cohort tool also gave real-time feasibility feedback showing the total matching population and a run-rate based on data. We had to dial in our database optimization for very fast response times here. This gave incredibly useful real-time feasibility feedback on cohorts for specimen requests and level-set expectations for time to collect specimens, and became a very powerful sales and project setup tool, removing a lot of the manual back-and-forth with a researcher.
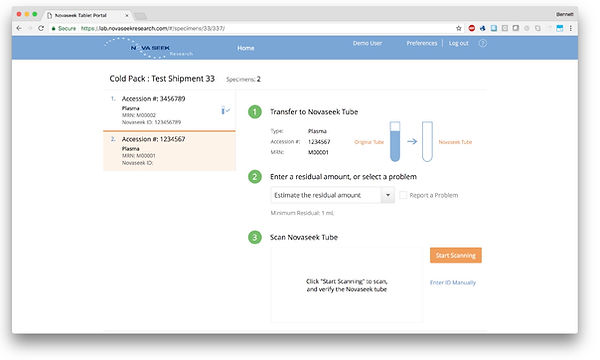
Workflow
The lab workflow and logistics were complicated. Lab staff had to pull a specimen just before it was discarded, pipette into a new container without PHI, then print a shipping label and manifest before packaging and taking the box to a FedEx pickup area. All labs do “send out”; sending specimens to reference labs for tests that aren’t run in-house. We piggy-backed on this process to minimize impact on the lab staff. In addition, we designed our lab UI/UX to flexibly accommodate any workflow variant for shipment preparation. This included specimen handling, specimen preparation, batching, and special shipping instructions. To further reduce burden on lab staff, we integrated with FedEx to dynamically produce the shipping label and the manifest (required by IATA regs). We conducted time-and-motion studies with Applied Management Systems (lab consultants) to demonstrate a 10 minute average time to prepare a shipment for Novaseek. All of our supplier labs found the lab tools and workflow for Novaseek to be easy to understand and use.
EMR Integration
This program used a wide array of structured and unstructured data. We created generic EMR specific reports (NPR reports for Meditech, CCL reports for Cerner) that included 95% of the work needed at each site. We also supported HL7, but most sites opted to use the reports with a few last-mile modifications to tailor to the site. This allowed us to very quickly deploy EMR integrations at a low cost with our fastest being 6 weeks from start to finish. As FHIR adoption increased we started using FHIR as the canonical data format in our own back-end but did not find wide enough utilization at hospitals to ever make the complete switch.
Regulatory Compliance
This product had to comport with multiple regulatory regimes including HIPAA, NIH Common Rule, and IATA regulations, with a nod to FDA part 23 data chain of custody for regulated diagnostic validation studies. We operated under an umbrella IRB protocol and all technology and operations had to align to the protocol. This included administering ICFs where applicable and working with data under waiver of consent. Additionally, we had to become conversant in research regulations because most hospitals required some education as part of the sales process. We never had any breaches or reportable adverse events, and we never lost a sale to a hospital due to security/privacy concerns.
Informed Consent
We initially attempted to obtain a consent from every patient whose remnant specimens we collected. While this added some additional value to the specimens, we discovered that not all projects required consent and this greatly limited our access to specimen supply. I led a revision of our IRB and technical program that allowed us to access essentially every patient at the hospital under waiver of consent, increasing the number of specimens we could access by ~20x-30x, and allowing us to supply larger orders faster.
Supplier Selection
Selecting good hospital partners was essential to our success. Unfortunately several of our hospitals were financially unstable and either sold or filed for bankruptcy protection within the first year of our supplier contract with them effectively ending our relationship. This led to a lot of wasted effort implementing and training the sites and ultimately significantly hindered our ability to serve specimens to researchers reliably. We created a supplier qualification form that covered technical and lab operational issues, but failed to adequately qualify organizational stability. This is an area I would approach differently based on this experience.
Technology
-
Postgres data warehouse for ~10M patient records
-
Java/Quarkus REST API
-
Angular front-end with lab logistics workflow and real-time cohort feasibility
-
Azure, GCP, and AWS cloud deployments using Kubernetes
-
Fully automated multi-cloud CI/CD
-
Algorithmic matching of patients/specimens to research requests using both structured data and unstructured clinical narratives with NLP
-
EMR integrations with Epic, Cerner, and Meditech